Unique Device Identification (UDI) was implemented by the U.S. Food & Drug Administration (FDA) to track and identify medical devices within the United States. According to Thomas Gross and Jay Crowley, authors of Unique Device Identification in the Service of Public Health, the FDA has a process called the 510(k) which involves new medical devices being cleared and approved just based on being equivalent to other previously cleared devices (Gross & Crowley 1584). This means that if a new medical device is trying to enter the market it can get approved if it is modeled after another medical device without any significant clinical trials. To offset this process as well as keep up with other complications, the FDA wanted to strengthen the national system for medical devices. The FDA then signed into act on September 27, 2007, that all medical devices would require a unique identifier that would be assigned during distribution and tracked throughout the use of the particular medical device after insertion. In order to start this process, the FDA had to first come up with a system to develop these unique identifiers, make sure that there was a way to track the device during insertion, and also maintain a database to track the device after insertion. The FDA has three main agencies that are over UDI issuing which include: GS1; Health Industry Business Communications Council (HIBCC); ICCBBA. These agencies are responsible for upholding certain international standards, regularly apply for renewal, and fulfill the goals of the UDI program. Medical device companies that want a new device approved will have to go through one of these agencies and follow their guidelines.


Gross and Crowley describe the future of the UDI system when they say, “It will permit more accurate and timely reporting and analysis of adverse events, which will help the device industry, health care facilities, and regulators to more quickly identify and address problems relating to a particular device” (Gross & Crowley 1584). This was related to a situation that happened in May 2011, where some medical equipment got stolen including: endoscopy devices, urology devices, clipping devices, pelvic-floor repair kits, and implantable mesh slings. These stolen devices entered medical facilities claiming to be sterile when in fact the equipment was not sterile. Clinicians found that it was difficult to identify which ones were contaminated and which ones weren’t without the UDI system. Per the FDA’s website some other benefits of the UDI system are, “Enhancing analysis of devices on the market by providing a standard and clear way to document device use in electronic health records, clinical information systems, claim data sources and registries (…) A more robust postmarket surveillance system can also be leveraged to support premarket approval or clearance of new devices and new uses of currently marketed devices” (www.fda.gov). UDI specific labels for a device has certain requirements such as: manufacturer, make/model, description of some sort, packaging level/number of items, country of origin, single use or reusable, sterility, and storage conditions. By putting all of this information in a database, they will be able to track anything that occurs with the device.
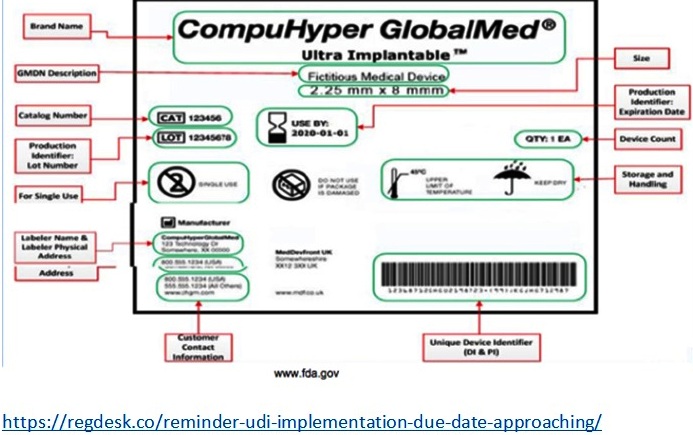
The FDA had to go through several different phases in order to implement the UDI process. First, they had to develop the UDI, then create an UDI application process, and lastly implement the database.
RegDesk, a company that provides applications and regulatory insights about medical devices, talks about the announcement of UDI when they state:
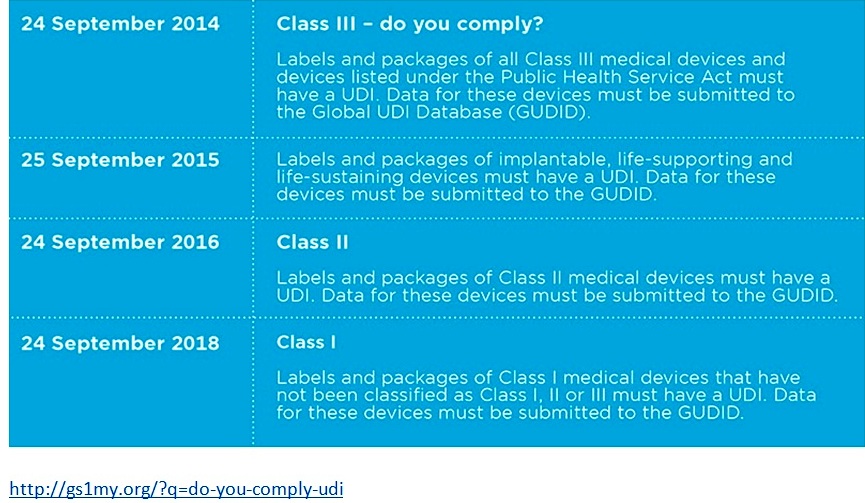
Currently, all Class III and implantable, life-supporting and life-sustaining medical devices require UDI’s. The next phase of the system will be implemented by September the 24th, 2016. After this date, all Class II medical devices will legally require UDI labels before product distribution. Additionally, Class III devices and PHS-licensed devices intended for certain uses will require direct marking. (Regdesk.com)
Class II and Class III devices are considered to be at moderate risk therefore they all require UDI as of now. In order to get your medical device approved for UDI, you must apply to one of the three government agencies mentioned earlier. The application process involves reading and filling a specific application depending on which agency you go through. They will then send you an approval letter or in some cases will send an accreditation extension letter. Class I devices are also going to be implemented for UDI in late 2018.
Some problems that are emerging within the UDI industry are having companies get the facts straight. Companies don’t know whether their brand manufacturer (a company that uses a second company to source out its own branded product) or their contract packager (a company that packages products for their clients) are responsible for the execution of UDI. Craig Hodgson, an author on Loftware.com, goes into further detail about this problem when he states, “It gets complicated quickly, which begs the need for an integrated, automated labeling solution that draws from sources of record to improved accuracy at every stage of the supply chain” (Loftware.com). Here, he suggests that there should be some sort of actual process that each company has to comply by whether it is through the brand manufacturer or the co-packager. Therefore, only a certain one needs to be set in place to be accountable of UDI. Another problem UDI implementation is having is communication with companies that still have yet to conform. Hodgson goes on to say, “Scary to think that at this stage, there’s not industry-wide awareness, but evidently there are pockets where the word is still not getting out” (Loftware.com). He is speaking in regards to when a large grocery company’s pharmaceutical department wasn’t aware of the UDI requirements. It is said that the best way to get UDI started within your organization you should include every department such as: regulatory, manufacturing, supply chain, and IT.

UDI can be very helpful and innovative to the medical device world. An analogy to compare it to would be the automotive industry. They assign VIN numbers to each car which tracks this car throughout the duration of its lifetime. Meaning any recalled parts, anything that breaks, and tracking the owners of the vehicle can be traced. This is helpful to provide important information for that particular vehicle. UDI is essentially the same but instead of a car it will be that specific device tied to a patient. This will then be able to extend patient care and innovate the industry further.