COVID-19, also known as Coronavirus, has been an unexceptional problem and talking point in our everyday lives for the past year. As we start to get used to following the safety protocols and requirements required for the containment of the spread of the virus, the development of a vaccine for the virus is in the works and is beginning to be given to thousands of people. With that in mind, the big challenge now is how these vaccines will be received by the public since the production of it is reserved to only a couple of locations worldwide which may make the distribution process more complicated.
The COVID-19 virus is a newly discovered virus that attacks respiratory channels. Although the World Health Organization says that most of the people infected by the virus will experience mild to moderate symptoms, the virus spreads very quickly and can be problematic for older people, people with chronic respiratory problems, or those who are going through a tough disease already (World Health Organization).
The Coronavirus has found its way into packaging since the first couple of months of the pandemic. One of the first concerns that Americans had about online shopping was the fact that most of the products are produced and packaged in Asia before they are shipped to the United States, which could potentially be a risk of spreading the virus over on this side of the Pacific. However, that fear did not last long since E-Commerce has never been used so much by the American shopper. Now, many people like the fact that there is no direct contact with any other person when making an online purchase. Instead of going out to the grocery store or an appliance store to buy what is needed, people now look online first. There are many protocols of packaging that have been addressed during this time of uncertainty caused by the pandemic.
Vaccine Packaging
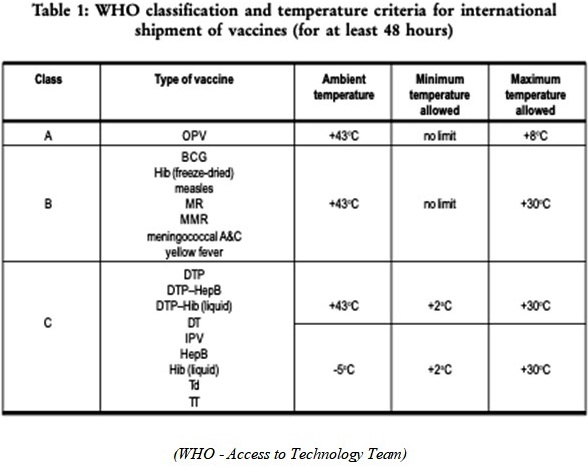
There are three classes of packaging for vaccines, and they all differ on the insulation level and the internal temperature of the package that the vaccine must be kept in. Class A is for the coldest temperatures where the vaccine must be kept in a very cold temperature, maximum temperature allowed inside the insulated package is 8oC. Class B is for the vaccines that can be kept at a max temperature, inside the insulated package, of 30oC. Class C is the one that the warmest temperature must not exceed 30oC, but it also cannot be lower than 2oC, inside the insulated package. These classifications are recommended for vaccines that will be in the process of shipping or transport for at least 48 hours. (WHO - Access to Technology Team)
The current main issue with the most advanced vaccine at the moment, developed by Pfizer, is the fact that it has to be transported at an extremely cold temperature; it has to be transported and stored at around -70oC (-94oF) (Ducharme). These extreme temperatures are not only a challenge to the companies transporting and storing the vaccine but also a huge challenge to the engineers that are developing a package that is able to insulate the vaccines at such low temperatures. These vaccines do not fit in any of the current guidelines established by the WHO, and because of that, the already built and developed infrastructure is not a fit for these vaccines.
The Plan
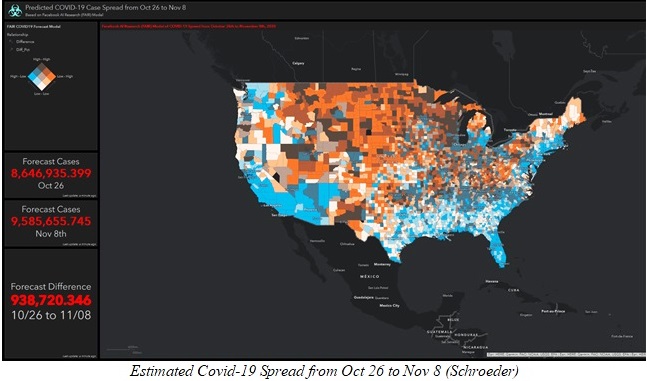
The Corona Virus pandemic is now part of our lives and it does not look like it is going anywhere anytime soon. Many pharmaceutical companies have been on the chase for a vaccine that will help us get over this new virus, and now with the winter on our doorstep the need for an efficient way to contain it is of extreme necessity. The number of infected people is back on the rise and as of October 29, 2020, nine million people has contracted the virus, and unfortunately about 230,000 people has died from it, since the beginning of the pandemic (Schroeder). One of the most efficient vaccines developed today is the one by the pharmaceutical company Pfizer. Pfizer’s vaccine has been proven a high percentage of effectiveness and is already been produced by the pharmaceutical giant. Pfizer’s vaccine is more than 90% effective (Reuters Staff) which is something amazing and that will be able to save many lives in the long run if a big percentage of the world gets vaccinated as soon as possible. Pfizer has been developing a packaging system able to hold the low temperatures required by its vaccine for days, but the fact that the vaccine has to be constantly kept at about -70oC is a huge problem when trying to vaccinate 5 billion people.
Pfizer currently has two big cold storages located in Belgium and in Michigan in the United States (Robbins e Gelles). These two facilities are enough to supply the vaccine for thousands of people, but not enough to solve the main problem of the rest of the world, which is consequently our problem too. If just a small part of the world gets the vaccine this virus will not get eradicated, then the chances of mutations and consequently another virus outbreak is great.
Another main Pharmaceutical company on the chase for a vaccine is Moderna. As of today, Moderna has developed a very safe vaccine that can be maintained at more reasonable temperatures than the one developed by Pfizer, but the catch is that it is not proven to be as effective. The Moderna vaccine vaccine has to be kept at around -20oC (-4oF), it still is very cold, but there are airplanes that are equipped for shipments at temperatures below -25oC (Muller), but the main freight of airplanes that can keep such temperatures are owned by the German airline company Lufthansa.
The packaging Strategy
The problem is not solved even with all these airplanes and cargo storages that can keep the vaccines at such low temperatures. The real big problem is to keep these vaccines at the required temperatures when it is time to vaccinate the population of a country with millions, if not billions of people. The fact that we already have developed vaccines with such high efficacy is something unprecedented, but it will not be of any help if not everybody gets vaccinated. Hospitals in big cities are rushing to get the proper freezers to keep the vaccine, but the rural hospitals do not have the money or even the infrastructure to get these ultra-cold freezers that can cost up to $15,000. After a more temperature reasonable vaccine is developed, these freezers will most likely not be any help for the regular vaccines that can be kept at a little over freezing temperatures (Goldhill).
Pfizer has been developing packages that can hold one thousand to five thousand doses and potentially keep the ultra-cold temperatures for about 10 days. This packaging will be made of a super insulated material on the inside that will withhold the doses. The outside will most likely be made of cardboard and insulated foam containers to keep the doses cold and packaging costs to a minimum. These doses will be stacked in a pizza box style package surrounded by dry ice. This package will be able to keep the vaccines cold enough for about 5 days after it is opened, which then will require a quick replacement of dry ice (Goldhill). The packaging also has another major limitation. It can only be opened for one single minute and that cannot be done more than two times a day. Those last two limitations mean that the places keeping the vaccines will have to take a wild estimation of how many people will get vaccinated each day and extract that exact amount as fast as possible from the box to then be kept at a super cold freezer until that vaccine is used. That means that the distribution center will have to accurately predict how many doses they will give each day before that specific day. If too many doses are taken out of the box, those lifesaving vaccines will be wasted. However, the process will be delayed by not vaccinating everyone they can if not enough doses are taken out of the box.

The Box
The packaging undoubtedly needs improvement if the ultimate goal we are reaching for is vaccinating as many people as possible as fast as possible to get this virus outbreak under control. Engineering a container to hold many doses at such temperatures will not be easy, but it certainly can be done if we want to pay a premium for these vaccines. Super insulated boxes is something that can easily be done, but taking that to a super scale is where it gets hard; super insulated packaging is easy to make when it is done for one product only, but it surely is more expensive if compared to a regular container. Companies like FedEx and UPS are already getting ready for the super cold vaccines. FedEx is adding ultra-cold freezers in strategic locations such as Memphis, Indianapolis, and Paris; as well as installing refrigerated trailers in Oakland, Los Angeles, and Dallas (Gelles).

UPS is building something that they call the “Freezer Farm” in Louisville, Kentucky, the location of the company’s largest hub. There they will be able to store millions of doses that will be able to be shipped all over the United States in a matter of hours. The same style of warehouse is also in development by UPS in the Netherlands (Gelles).
FedEx vaccine shipping crate during the 2009 H1N1 pandemic. (Gelles)
The scale of this operation is gigantic, something that no other generation has ever encountered, which means that there is a lot of pressure for us to take this situation seriously and steadily so that we do not jeopardize the next generations to come after us. We are in unprecedented times, and during these times unprecedented actions must be taken to ensure the safety of all of us and those in the future. We must work together to find ways to overcome this situation for a better future.
References
Ducharme, Jaime. You might not be able to get Pfizer's frontrunner Covid-19 vaccine. 13 November 2020. November 2020. <https://time.com/5911543/pfizer-vaccine-cold-storage/>.
Gelles, David. How to Ship a Vaccine at –80°C, and Other Obstacles in the Covid Fight. 18 September 2020. November 2020.
<https://www.nytimes.com/2020/09/18/business/coronavirus-covid-vaccine-cold-frozen-logistics.html>.
Goldhill, Olivia. ‘We’re being left behind’: Rural hospitals can’t afford ultra-cold freezers to store the leading Covid-19 vaccine. 11 November 2020. 12 November 2020. <https://www.statnews.com/2020/11/11/rural-hospitals-cant-afford-freezers-to-store-pfizer-covid19-vaccine/>.
Muller, Joann. Vaccine offers hope for airlines — and a big logistical challenge. November 2020. November 2020.
Reuters Staff. Cold storage challenges could hamper distribution of Pfizer, Moderna COVID-19 vaccines - Fauci. November 2020. November 2020.
<https://www.reuters.com/article/health-coronavirus-vaccines-distribution/cold-storage-challenges-could-hamper-distribution-of-pfizer-moderna-covid-19-vaccines-fauci-idUSKBN27R2EB>.
Robbins, Rebecca and Davis Gelles. How Pfizer Plans to Distribute Its Vaccine (It’s Complicated). 12 November 2020. The New York Times. November 2020. <https://www.nytimes.com/2020/11/12/business/pfizer-covid-vaccine-coronavirus.html>.
Schroeder, Andre. As Covid-19 Cases Rise Again Across the United States, Where Should We Be Most Concerned? 29 October 2020. November 2020. <https://www.directrelief.org/2020/10/as-covid-19-cases-rise-again-across-the-united-states-where-should-we-be-most-concerned/>.
WHO - Access to Technology Team. Guidelines on the international packaging and shipping of vaccines. Guideline Brochure. World Health Organization. Geneva, Switzerland: World Health Organization, 2005.
World Health Organization. Corona Virus - WHO. 2020. 9 November 2020. <https://www.who.int/health-topics/coronavirus#tab=tab_1>.